Medgadget Editors Anesthesiology, Critical Care, Emergency Medicine, Medicine, Surgery
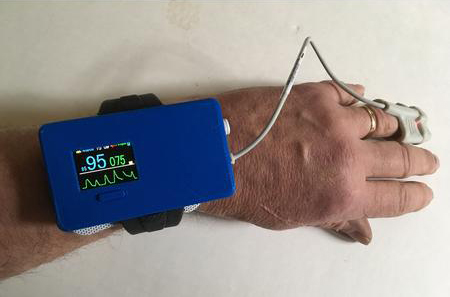
Med-botics, a firm based in Colorado Springs, won FDA Breakthrough Device status for its Oxalert EPO (Enhanced Pulse Oximeter), a device developed to prevent respiratory arrest from opioid overdoses.
Post-op patients and others on heavy opioid therapy can stop breathing, which can lead to death if not monitored carefully. The Oxalert EPO monitors patient SpO2 (oxygen saturation) levels and if those fall below 90%, the device attempts to arouse the patient using audible notifications via headphones, gentle electric shocks, or a combination of the two.
The device is worn on the wrist, much like other pulse oximeters. This one is larger in size because of its active functionality.
Behind this device is a proven concept evaluated during a study at Oregon Health Science University eight years ago that demonstrated that “a continuous pulse oximeter, headphones for verbal prompting, a nerve stimulator, and a laptop computer automatically prevented hypoxemia (oxygen levels below 90%) in heavily-sedated patients emerging from surgery,” according to Med-botics.
The new Breakthrough Device designation doesn’t mean that the device is approved for use in the United States yet, but makes sure that it’s rushed through the regulatory approval process.
Product info page: Oxalert EPO (Enhanced Pulse Oximeter)
Via: Med-botics
